Event date:
Sep
10
2021
3:00 pm
Development of Self-supported 3D Porous Metallic Electrocatalysts for Chemical-assisted Energy-saving Hydrogen Production
Supervisor
Dr. Falak Sher
Student
Farhan Arshad
Venue
Zoom Meetings (Online)
Event
PhD Synopsis defense
Abstract
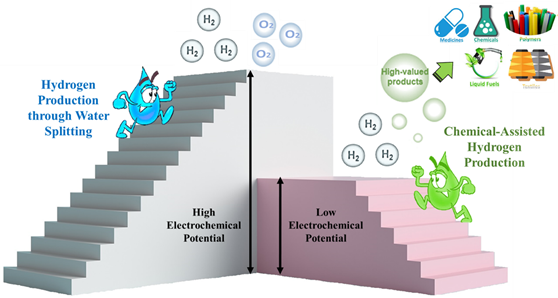
Hydrogen gas is regarded as a sustainable, environment-friendly, and promising source of energy for the future. Water electrolysis is a very attractive process for the production of H2 in which two half-reactions are involved: oxygen evolution reaction (OER) and hydrogen evolution reaction (HER). Out of the two half-reactions, OER requires higher electrochemical potential due to the involvement of the four-electron transfer mechanism. Water splitting is a well-established technology, but still, there are several limitations for the large-scale production of hydrogen using this process. Firstly, the most important factor is the OER process that requires more electrical energy. Secondly, the unavoidable mixture of oxygen gas with hydrogen gas may propel the reaction to explosion in the electrolyzer. Several approaches have been suggested to reduce energy consumption and avoid the possibility of explosion. Chemical-assisted electrocatalytic water splitting is a cost-effective approach for replacing OER with a thermodynamically favorable anodic oxidation reaction in water. CAHER's process has recently moved towards a more competitive method using the biomass-derived intermediates as reductive chemicals to get hydrogen fuel along with valuable products. Electrochemical oxidation of methanol, some biomass-derived alcohols (ethyl alcohol, benzyl alcohol, and furfuryl alcohol), and carbohydrates (glucose, fructose, maltose, and sucrose) show not only high performance for hydrogen production but also produce high-value products. To date, noble metals such as Pt, Pd, Ir, Ru, and their alloys are the most efficient catalysts for the chemical oxidation in the CAHER system. Several critical challenges in the development of CAHER still need to be addressed such as the issue of low catalytic selectivity and efficiency toward the most valuable products in alcohol electrooxidation reactions. In this regard, we have synthesized 3D hierarchical nanostructured NiCo porous bimetallic foams on Cu foil electrodes by a simple, ultrafast, and single-step bubbles templating electrodeposition method. Benefiting from the excellent electronic conductivity, high internal reactive surface areas, profiting electrolyte accessibility, and effective mass transfer at the electrolyte/electrode interface, NiCo foam exhibited excellent activity for HER, OER, and high selectivity for methanol oxidation to value-added formate product in alkaline methanol-water solution. Furthermore, the integrated two-electrode electrolyzer required a cell voltage of 1.67 V for overall water splitting and 1.47 V for the overall methanol-water solution at a current density of 20 mA cm-2 with continuous 12 h of operation without obvious decay.
Meeting Link: https://lums-edu-pk.zoom.us/j/94417593994?pwd=VlNvc3Y2ekFUWVNyMFRjcmxOVmxodz09
Meeting ID: 944 1759 3994
Passcode: 126058

