Event date:
Aug
13
2021
3:00 pm
Electron Donor Acceptor Double Cable Covalent Conjugates: Towards Improved Photovoltaic Devices and Accelerated Artificial Photosynthesis
Supervisor
Dr. Basit Yameen
Student
Sunniya Iftikhar
Venue
Zoom Meetings (Online)
Event
PhD Research Seminar
Abstract
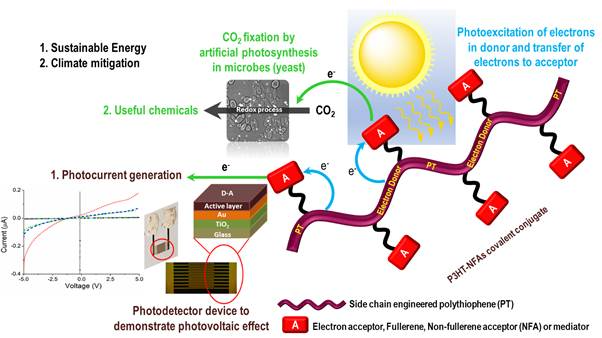
Tremendous amount of effort is being invested in improving the optical and electrical properties of semiconducting materials for use in diverse application domains such as photovoltaics, thin-film transistors, interfacing electrodes, surface coating, and biosensing devices. These efforts have led to a remarkable development in the field of semiconducting materials offering versatile chemical and functional traits. In this context, various chemically and functionally distinct classes of organic semiconducting polymers (OSPs) have been developed as alternatives to inorganic semiconductors. Compared to their inorganic counterparts, OSPs are easy to synthesize and present possibilities of installing chemically diverse functional groups on the polymer molecules as side chains and end groups. These provisions have resulted in the development of a toolbox of OSPs with tunable optical and chemical properties that can be customized for a target application. In quest of expanding the advantages offered by OSPs and in pursuit of novel OSPs with unique chemical, optical and electronic properties, we are developing a variety of side chain engineered OSPs for application in energy, and environment domains. In this seminar, we are presenting a side chain engineered polythiophene copolymer based OSP with pendant aldehyde groups. The target polymer was synthesized by a combination of Grignard Metathesis (GRIM) polymerization and post-synthetic modifications. The pendant aldehyde groups of OSP (electron donor-D) were covalently conjugated to the pristine C60 (elector acceptor-A) via 1,3-dipolar cycloaddition reaction (i.e., Prato reaction) between the pristine C60 and azomethine ylide formed in situ as a result of the reaction of pendant aldehyde groups and sarcosine. The OSP with pendant aldehyde groups and its covalent conjugate with pristine C60 were fully characterized using FTIR and 1H-NMR spectroscopies. Fluorescence spectroscopic analysis of the synthesized D-A double cable covalent hybrid and physical hybrid derived from the physical mixing of synthesized OSP and C60 revealed a higher fluorescence quenching in case of the covalent hybrid. This novel precisely designed D-A hybrid material was employed as the photoactive layer in interdigitated gold electrodes based photodetector where a significant enhancement in photocurrent (>2.5 times) was observed in the devices consisting of photoactive layer derived from the D-A covalent hybrid. This work offers a step forward in the systematic development of D-A covalent hybrids with a precise molecular design for the fabrication of efficient photovoltaic devices. Besides developing D-A covalent hybrids of OSPs and graphitic carbon nanomaterials, we are developing novel light harvesting systems based on covalent conjugation of OSPs with flavin (a non-fullerene acceptor - NFA) moieties are being developed as light-harvesting material for photosensitization of carbon dioxide (CO2) capturing and transforming microbes that work via a non-photosynthetic approach. The outcomes of this effort will help in finding alternative solutions to capture and convert CO2 into useful chemicals. In summary, this work will lead to the development of a diverse range of side chain engineered OSPs with precise molecular design that will enable fabrication of a variety of functional materials for energy, environmental, and biological applications.
Zoom Link: https://lums-edu-pk.zoom.us/j/92655980171?pwd=cnk0YnNWeTUzZDd2MWJRaTVKMjlNUT09
Meeting ID: 926 5598 0171
Passcode: 257834

