An Investigation of the Crystal Structure and Ionic Pathways of the Hexagonal Perovskite Derivative
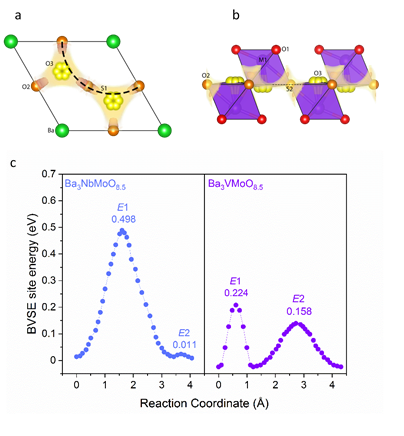
The study of oxide ion conductors has recently gained a great attention due to their use in electrolytes for solid oxide fuel cells (SOFC) and solid oxide electrolyser cells (SOEC), oxygen separation membranes, oxygen sensors and oxygen pumps. Currently, SOFCs require high temperatures (> 700 °C) to operate efficiently. The high operating temperature lead to poor durability of components, increased costs, slow start up times and issues with selecting compatible materials 1-2. There is a significant amount of research being carried out into developing oxide ion conductors that can operate at intermediate temperatures (400-600 °C). Numerous crystal systems including fluorite and La2Mo2O9 (LAMOX) systems, silicon, and germanium apatites, Bi-based compounds (such as BIMEVOX and BICUVOX) and perovskite oxides have displayed fast oxide ionic conductivity. Substantial oxide ionic conductivity has recently been reported in cation-deficient hexagonal perovskite Ba3M’M’’O8.5 derivatives, with disordered hybrid 9R-palmierite average structures,3-4 opens a new avenue to search novel oxide ionic conductors within this family. We have synthesised the hexagonal perovskite Ba3VMoO8.5 and elucidated its crystal structure. Rietveld refinement from neutron and X-ray diffraction data show that like Ba3VWO8.5, the cation vacancies are ordered on the M2 site leading to a structure consisting of palmierite-like layers of M1Ox polyhedra separated by vacant octahedral layers. In contrast to other members of the Ba3M’M’’O8.5 family, Ba3VMoO8.5 in not stoichiometric and both barium and extra oxygen vacancies are present. Ba3VMoO8.5 is unstable in air above 400 °C, which precludes measurement of the electrical properties. However, bond-valence site energy (BVSE) calculations strongly suggest that oxide ion conductivity would be present in Ba3VMoO8.5.
Zoom link: https://lums-edu-pk.zoom.us/j/98627138734?pwd=NHNHN3RBOGlOSlFpM250Vk5Yc2MvZz09
Meeting ID: 986 2713 8734
Passcode: 349394
References
- Jacobson, A. J., Materials for solid oxide fuel cells. Chemistry of Materials 2009, 22 (3), 660-674.
- Adams, T. A.; Nease, J.; Tucker, D.; Barton, P. I., Energy conversion with solid oxide fuel cell systems: A review of concepts and outlooks for the short-and long-term. Industrial & Engineering Chemistry Research 2012, 52 (9), 3089-3111.
- Fop, S.; Skakle, J. M.; McLaughlin, A. C.; Connor, P. A.; Irvine, J. T.; Smith, R. I.; Wildman, E. J., Oxide ion conductivity in the hexagonal perovskite derivative Ba3MoNbO8. 5. Journal of the American Chemical Society 2016, 138 (51), 16764-16769.
- McCombie, K.; Wildman, E.; Fop, S.; Smith, R.; Skakle, J. M.; Mclaughlin, A., The crystal structure and electrical properties of the oxide ion conductor Ba 3 WNbO 8.5. Journal of Materials Chemistry A 2018, 6 (13), 5290-5295.

