Event date:
May
19
2021
12:00 pm
Development of Self-Supported and Surface Etched Ni(Oh)2 as a Bifunctional Electrocatalyst for Overall Water Splitting
Supervisor
Dr. Habib ur Rehman
Student
Fatima Tahir
Venue
Zoom Meetings (Online)
Event
MS Thesis defense
Abstract
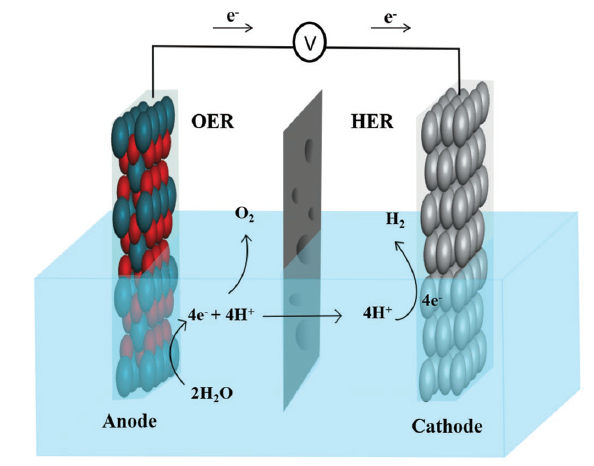
With the increasing world population, energy demands are constantly rising. Currently, over 80% of energy requirements are being met by the burning of fossil fuels. Fossil fuels have long been identified as a major cause for global warming and other environmental impacts thus their percentage in the energy mix needs to be drastically reduced and replaced by green and renewable resources. In recent years, water splitting has received huge attention as a promising clean and renewable energy resource because of its availability, environment friendly emissions, cost and feasible storage factors. Out of the two reactions (HER and OER), OER is considered as the bottleneck due to its slow kinetics and large overpotential. Precious metals such as Ru, Ir and Pt have shown good catalytic activity for OER and HER, however, their higher cost and limited abundance have discouraged their widespread use in commercial applications. In addition, catalysts based on these precious metals are prone to catalytic poisoning that seriously impact their long-term stability under high anodic potentials. For a largescale economical water splitting, catalysts that use earth abundant metals materials were developed but their complex, multistep and expensive synthesis remained a problem. So addressing this problem, in this research, we have reported a facile and cost-effective path to generate high-performance bifunctional Ni(OH)2@nickelfoam (NF) electrocatalyst by corrosion engineering. By optimizing different parameters such as acids (H2SO4, HCl, HNO3) corrosion, temperatures (room temperature (RT), 80°C) and concentrations we came up with two of our best catalysts. Acid washing of nickel foam promoted the dissolution of Ni+2 in solution and its reabsorption on NF as Ni(OH)2 nanosheets, which was further confirmed by Raman analysis and XRD, SEM and increased oxygen concentration in EDX plots. 2.4M HCl (RT) and 2.4M H2SO4 (80°C) washed samples exhibited over potential of 138mV@20mVcm-2 and 270mV@20mAcm-2 for HER and OER respectively in 1M KOH. During 12h test in 1M KOH within a two-electrode system, the cell exhibited excellent stability with a cell voltage of 1.61V@20mAcm-2.
Link: https://lums-edu-pk.zoom.us/j/93320055066?pwd=ZDFWcnRkbTgzWTJiekhEcGxaa0dHZz09
Meeting ID: 933 2005 5066
Passcode: 021605

