Event date:
Apr
9
2021
2:30 pm
The Relationship between Oxide-ion Conductivity and Cation Vacancy Order in the Hybrid Hexagonal Perovskite Ba3VWO8.5
Supervisor
Dr. Falak Sher
Student
Asma Gilane
Venue
Zoom Meetings (Online)
Event
PhD Research Seminar
Abstract
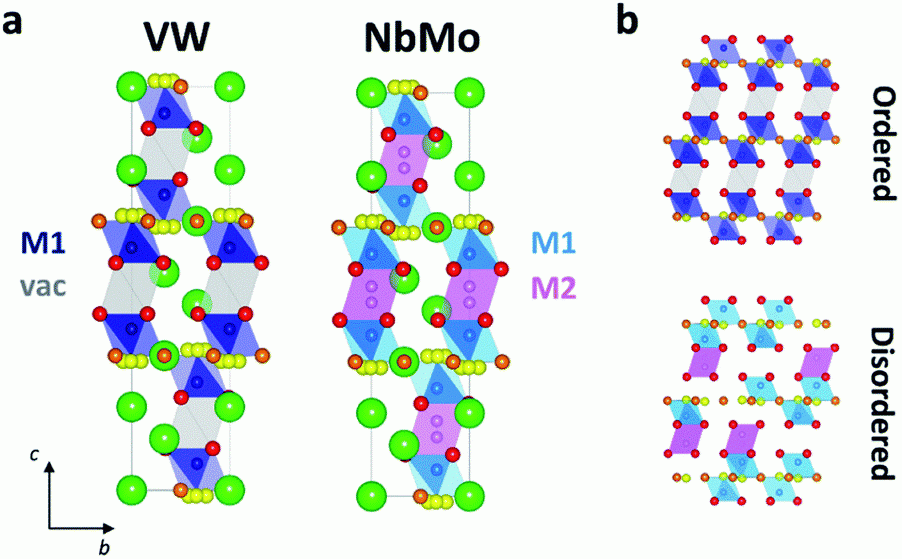
The development of sustainable, green, and efficient renewable energy conversion technologies is the major goal of many researchers these days, in order to meet the future energy demand and to reduce the environmental pollution.1-2 Fuel cells are the emerging electrochemical devices which can generate electricity from the renewable energy sources, with high efficiency ca. 60-80 % and low emissions of pollutants.3 Solid oxide fuel cells (SOFCs) are one of the most promising types of fuel cells having unique physiochemical properties i.e. fuel flexibility, tolerance to poisoning, noise free, use of cost-effective catalyst, heat and power combination which enhances their efficiency, and negligible electrolyte management.4-5 The high operating temperature is the major hurdle in the commercialization of SOFCs as it reduces the components’ durability, resulting in slow start up times and requires the use of expensive materials for seals and interconnects. Hence, it is desirable to develop novel electrolyte materials with enhanced oxide ion conductivity in the intermediate temperature range (300-650 °C) to overcome these issues.6 Significant oxide ionic conductivity has recently been reported in the cation-deficient hexagonal perovskite Ba3M’M’’O8.5 derivatives, with disordered hybrid 9R-palmierite average structures,7-8 opens a new avenue to search novel oxide ionic conductors within this family. We have synthesized phase pure Ba3WVO8.5 hexagonal perovskite derivative and investigated the crystal structure and electrical properties using various analytical techniques such as X-ray diffraction (XRD), neutron diffraction (GEM diffractometer, ISIS), Bond-valence site energy (BVSE) analysis and impedance spectroscopy. Electrical characterization demonstrates that Ba3VWO8.5 is also an oxide ion conductor with a total conductivity of 6.2 × 10-4 S cm-1 in air at 900 °C, thus revealing that it is possible to obtain oxide ion conducting Ba3M’M’’O8.5 materials with a variety of different M’M’’ combinations. While Ba3NbMoO8.5 and Ba3NbWO8.5 present a random distribution of cationic vacancies, X-ray and neutron diffraction experiments demonstrate that the cationic vacancies are ordered on the M2 sites in Ba3VWO8.5, resulting in a structure where M1Ox palmierite-like layers are separated by empty octahedral cavities. Bond-valence site energy (BVSE) analysis on the different phases demonstrates that ordering of the cationic vacancies hinders long-range oxygen diffusivity parallel to the c-axis in Ba3VWO8.5, thus explaining the reduced ionic conductivity of this compound.
Zoom link: https://lums-edu-pk.zoom.us/j/92364565188?pwd=aStGMTY1UFJSaUVHWDVoWWY1Q0wxQT09
Meeting ID: 923 6456 5188
Passcode: 576857

