Faster Oxide Ions, Higher Currents!
Not fun doing outdoor work on an extremely hot and humid day? We’re with you, and so is the oxide ion! But is it possible to change the ‘weather’ just because we don’t like it? Aha!!! That’s exactly what researchers at the School of Science and Engineering’s Chemistry and Chemical Engineering figured out. By changing the environment to help oxide ions move more comfortably, researchers can help generate electricity through a technique that’s more efficient, quieter and just plain better! Enter the world of Solid Oxide Fuel Cells (SOFCs)!
The whole process is as easy as 1…2…3!
1. Break up molecular hydrogen into H+ ions on one side.
2. Break up molecular oxygen into O-2 ions on the other side.
3. Generate electrons by allowing O-2 ions to travel through a dense electrolyte material from one side and combine with impatient H+ ions waiting on the other side. We get water (2H+ + O-2 -> H2O) as a by-product along with clean electricity.
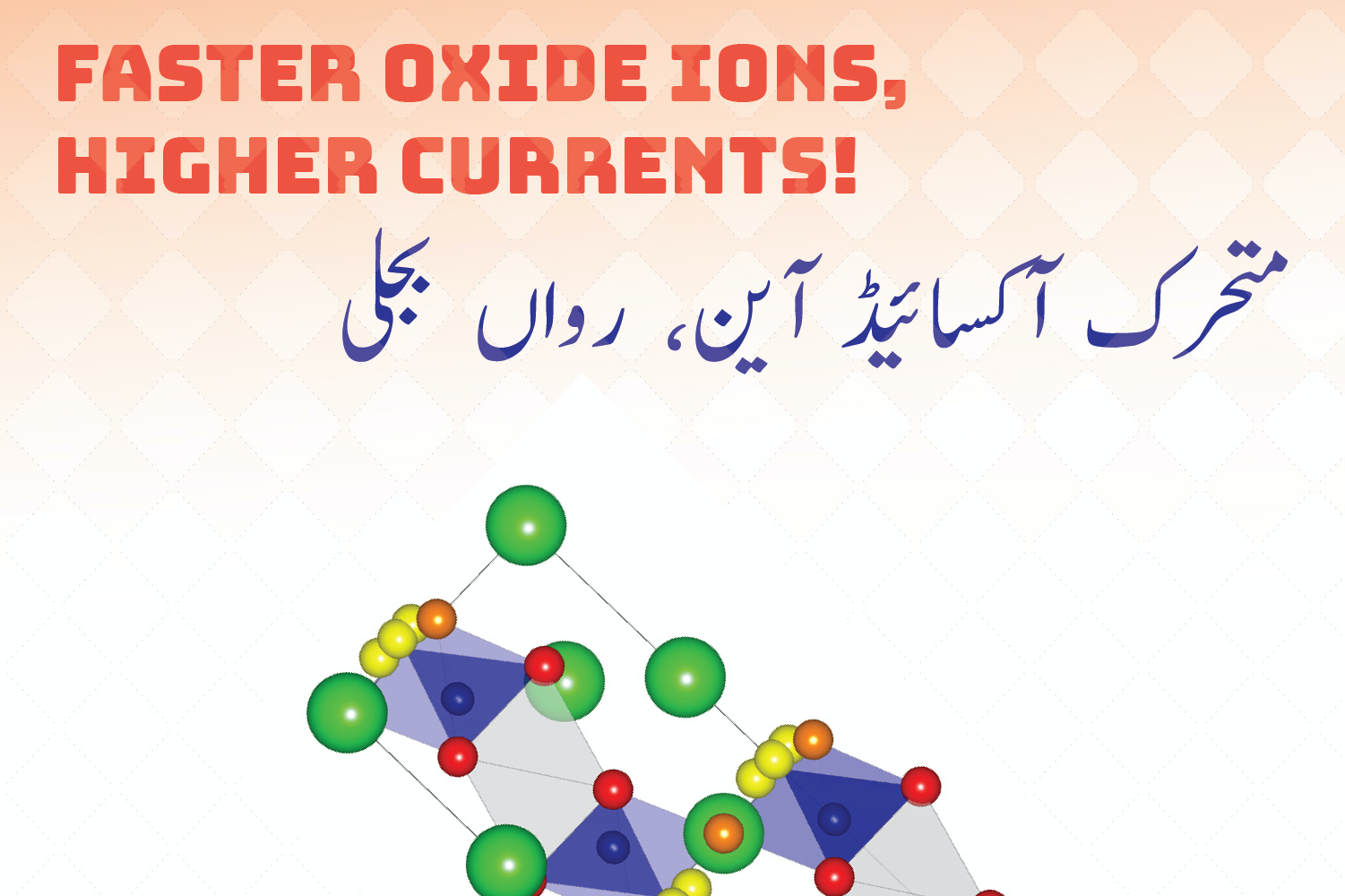
This seems like the best of both worlds! However, this is where the challenge begins. The key process by which SOFCs are able to generate electricity is the conductivity of oxide ions through the dense, solid electrolyte that sits between the electrodes. With current methods, the electrolyte needs to be at a temperature above 700 °C to enable good oxide ion conductivity, adding cost and complexity to SOFCs that render them difficult to commercialise. The research work highlighted in this article has discovered a different type of electrolyte that has good oxide ion conductivity even at lower temperatures of 600 °C. This may not sound like a big deal since we are still way above room temperature, but this is how scientific inquiry progresses, every little bit counts.
This research, supervised by Dr. Falak Sher and his PhD student Asma Gilane, who is a co-author, has revealed that the crystal structure of the hexagonal perovskite derivative (Ba3VWO8.5) exhibits electrical properties that render ultra-high oxide ion conductivity inside SOFCs. The key feature enabling this lies in the fascinating crystal structure, and is linked to the random and disorderly, yet highly connected distribution of oxygen-vacant and oxygen-occupied sites in the crystal, making continuous oxide ion migration possible even at lower temperatures. This is tantamount to creating the pathways for a smoother flow of traffic in a congested urban environment, the movers here are oxide ions and the alleys, streets and crossings are the conduction pathways for the ions.
The perovskites are a class of crystals that have enabled high-temperature superconductivity, magnetism, solar cells and ferroelectrics that we use in piezoelectric buzzers.
The work is published in Journal of Materials Chemistry A.
Reference: J. Mater. Chem. A, 2020, 8, 16506-16514 (DOI: 10.1039/D0TA05581F)
Link: https://pubs.rsc.org/en/content/articlehtml/2020/ta/d0ta05581f

