Development of Highly Efficient Self-Supported Transition Metal-Based Catalysts for Water Splitting
Abstract:
The ever-growing global energy demand and the associated environmental concerns of our current energy supplies have accelerated the search for new energy resources and conversion technologies that are sustainable, environmentally safe, low cost, and offer improved performance.1 Among the various options being explored, hydrogen is one of the most sustainable, environmentally benign, and clean fuel resources on the planet. Currently, more than 95% of the world’s hydrogen is being produced through steam reforming of fossil fuels and biomass. There is an urgent need for an alternative process for the synthesis of hydrogen that is cheap and uses carbon-neutral resources. Water electrolysis is an effective and decisive method to produce hydrogen fuel because it is an abundant, carbon-free, clean, and renewable energy source, however, the process to convert water into hydrogen (water electrolysis) is hugely challenging from a thermodynamic and kinetics standpoint as it requires a significant amount of energy (over 237.2 kJ mol−1) under normal operating conditions. Electrocatalysts have been used to lower kinetic barriers and enhance the energy conversion efficiency of this process. 2 Platinum, ruthenium, and iridium-based catalysts are most effective for water splitting. However, due to their low natural abundance, electrocatalysts from these materials are prohibitively expensive. Lots of work has been done to explore cheap materials as a replacement for rare-earth metals. 2 Although highly efficient transition metal-based catalysts have been reported, however, most of these catalysts involve either complex synthetic processes or lengthy protocols that add to the overall cost of these catalysts. There is a strong desire to synthesize efficient electrocatalysts through simple and uncomplicated processes that are not only cost-effective but also are simple and more sustainable.
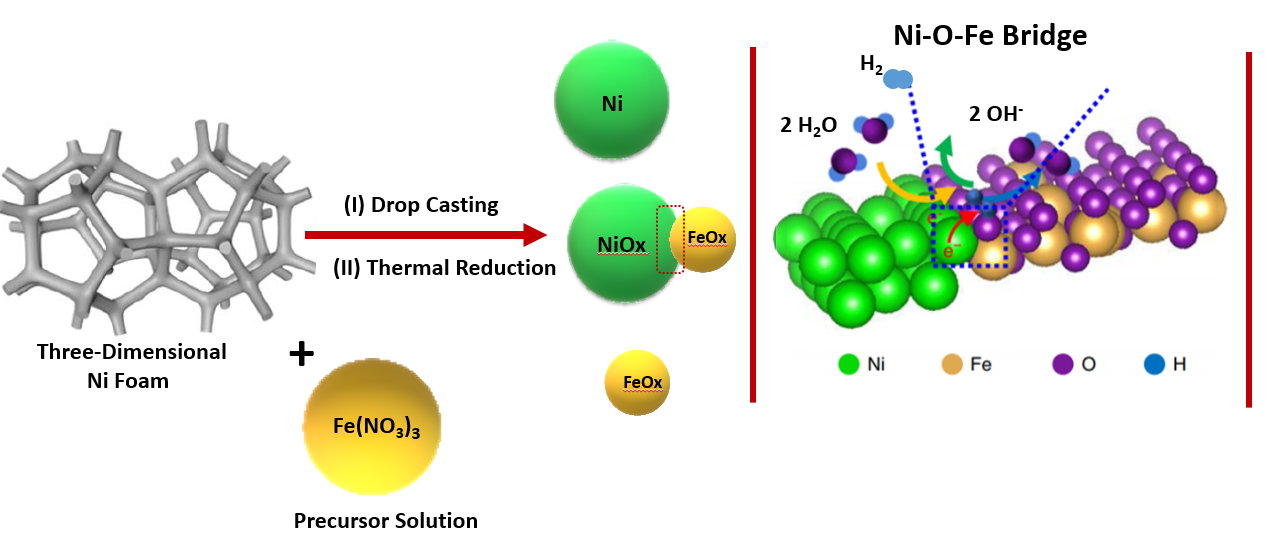
The main objective of this study is the development of facile and cost-effective synthetic protocols for the realization of efficient electrocatalyts for overall water splitting. In this work, highly efficient iron based electrocatalysts have been desinged and develpoed by directly depositing Fe on nickel foam by drop-casting methed. The best electrocatayst prepared through this route required an overpotential of 27 mV at 10 mA cm-2 for hydrogen evolution reaction, while for oxygen evolution reaction (OER) the overpotential of only 288 mV at 100 mA cm-2 was required. The prepared electrodes (Fe-NFAs-NF) show the excellent performance in overall water splitting with a cell voltage of 1.58 V at 20 mA cm-2. The enhanced electrochemical activity of these catalysts can be attributed to the large surface area, abundant active sites, impoved conductivity and efficient electron pathways. This research seminar will present the and discuss the major results of this project, the mechanisms involved in synthesizing the unique morphologies and the structure-property relationships for enhanced electrochemical performances. Overall, the strategies presented in this seminar are simple yet effective for making composite structures that can be further explored for the fabrication of other metal/metal oxides over nickel foam substrates with potential applications in energy generation, energy storage, and environmental remediation.
References
1. Suryawanshi, M. P.; Ghorpade, U. V.; Shin, S. W.; Suryawanshi, U. P.; Jo, E.; Kim, J. H., Hierarchically Coupled Ni: FeOOH Nanosheets on 3D N-Doped Graphite Foam as Self-Supported Electrocatalysts for Efficient and Durable Water Oxidation. ACS Catalysis 2019, 9, 5025-5034.
2. Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H. M., Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chemical Society Reviews 2017, 46 (2), 337-365.
Evaluation Committee
Dr. Habib ur Rehman (Supervisor)
Dr. Irshad Hussain (Thesis Committee Member)
Dr. Basit Yameen (Thesis Committee Member)
Dr. Salman Noshear Arshad (Thesis Committee Member)
Zoom Link: https://lums-edu-pk.zoom.us/j/91450600731?pwd=RXhRY21UUll1NkVTZDRPSVBmN…
Meeting ID: 914 5060 0731
Passcode: 116634

