Synthesis, Photophysical, and Electrochemical properties of novel imidazolone based compounds.
Abstract:
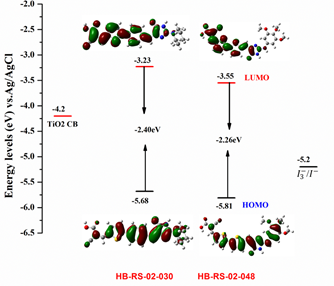
Two novel Phorbatopsin A (benzylidene-2-aminoimidazolones) derivatives based on donor-linker-acceptor (D-π-A) motif, HB-RS-02-030 and HB-RS-02-048 have been synthesized and characterized by standard spectroscopic techniques. The donor building block comprises (E)-4-benzylidene-2-(piperidin-1-yl)-1H-imidazol-5(4H)-one and (Z)-4-(thiophen-2-ylmethylene)-2-((3,4,5-trimethoxyphenyl)amino)-1H-imidazol-5(4H)-one while an electron acceptor side has cyanoacetic acid linked through a thiophene π-conjugated spacer. These novel metal free organic compounds were prepared by step wise approach from 2-(methylthio)-5-oxo-4,5-dihydro-1H-imidazol-3-ium iodide by using aldol condensation, nucleophilic substitution, Suzuki coupling and Knovenagle condensation reactions. Further, we have reported their photophysical and electrochemical properties thoroughly. HB-RS-02-030 showed absorption in violet region (λmax =436nm) while compound HB-RS-02-048 absorbed in blue region (λmax of 482nm). UV analysis of HB-RS-02-030 and HB-RS-02-048 also depicts bathochromic shift of 58 nm and 104 nm with respect to their parent molecule AH-RS-01-052 (λmax=378nm). The fluorescence spectra of both compounds exhibited orange emission (520-660nm). The HOMO/GSOP and LUMO/ESOP energies have been determined by using cyclic voltammetry. The observed stoke shifts (5392cm-1 and 3912cm-1) and band gap values 2.40eV and 2.26eV support thermodynamic feasibility for intramolecular charge transfer character from donor to acceptor moiety. The addition of a thiophene ring to the backbone of a molecule reduces the bandgap, allowing fine tuning of optoelectronic properties. HB-RS-02-030 absorbed higher with molar extinction coefficient 19200M-1cm-1 than HB-RS-02-048 which is found to be similar to N719 (141000 M-1cm-1) revealing its good light harvesting nature. The density functional theory (DFT) showed optimized geometries and frontier molecular orbital (FMO) energy levels that were found to be well aligned with corresponding experimental results. Our data suggests that these compounds could be considered as potential candidates for electronic and optoelectronic applications such as electroluminescent devices, field-effect transistors, photovoltaic devices, electro-optic modulators, and solid-state lasers.
Proposal Defense Committee:
- Dr. Falak Sher (Chemistry and Chemical Engineering/LUMS)
- Dr. Habib ur Rehman (Chemistry and Chemical Engineering/LUMS)
- Dr. Rahman Shah Zaib Saleem (Supervisor, Chemistry and Chemical Engineering/LUMS)
Meeting Link: https://lums-edu-pk.zoom.us/j/92779775960?pwd=RnF5V2NLdUxFbWU4TXNYTThTRWJhZz09
Meeting ID: 927 7977 5960
Passcode: 386121

