Doping Materials into Perfection
Harnessing solar energy is intricately linked with tinkering of molecular structure inside state-of-the-art materials. Coating traditional silicon panels with a layer of perovskite has boosted our hopes for a better, much more efficient way of generating electricity from solar energy.
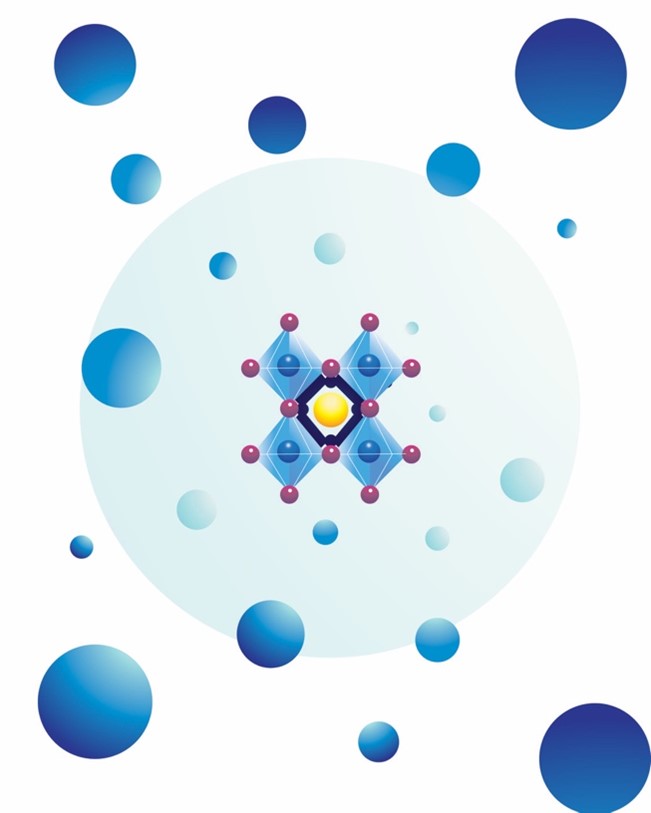
Pictured above is an artist’s rendition of what a perovskite crystal structure looks like.
However, it is not just light that perovskite is good at capturing. Let’s talk about heat – the greatest escape artist known to the physicist. Whether the process is chemical or physical in nature, or radioactive decay; heat always manages to escape into ‘the great outdoors’ of a given physical system. Wishful as it may be, imagine if this ‘wasted heat’ could be utilized to generate electricity, increasing efficiency of a thermoelectric system. Dr. Uzma Hira, a former student of Dr. Falak Sher (Chair, Department of Chemistry and Chemical Engineering, SBASSE) is the first author of a research paper that describes just that! A chemical doping process that can create a material which shows much better thermoelectric properties than conventional materials of similar kind. The answer is Hexagonal Double-Perovskite-Type Oxides, that substitutes Barium with Bismuth. The research paper entails how chemically doped Ba2−xBixCoRuO6 hexagonal double-perovskite-type oxides were prepared using a solid-state method and characterizes its interesting thermoelectric properties.
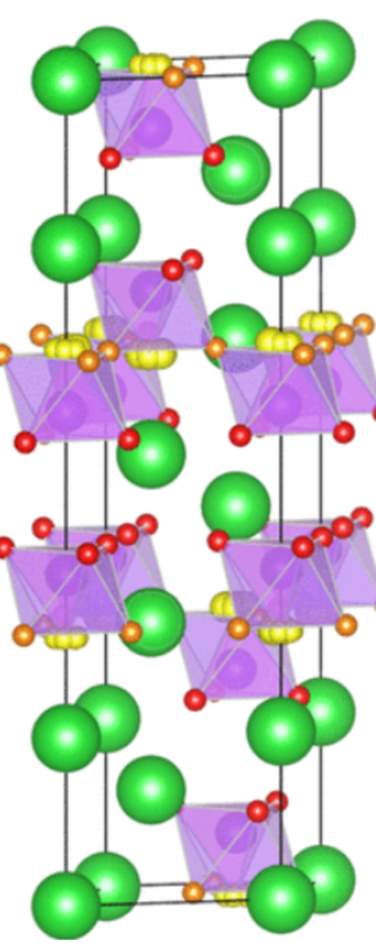
Shown above is the hexagonal interface of a double-perovskite type oxide, which offers promise as a p-type thermoelectric material. Pictured below is
Double perovskite oxides having the general formula A2BRuO6, where A is an alkaline-earth or rare-earth metal and B is a transition metal, show very interesting magnetic and electronic properties. The key parameter here is how effectively does a material demonstrate the Seebeck effect (named conspicuously after the Baltic German physicist, Thomas Johann Seebeck). In this fascinating phenomenon, temperature difference between two conductors can induce an EMF, generating a potential difference which can be measured. The material which Dr. Uzma’s team has worked on can be conveniently identified as Ba2CoRuO6, which is doped with Bismuth for better thermoelectric performance.
Conservative estimates suggest that about half of the total energy that we consume each year is lost to the environment as waste heat. Thermoelectric power generation offers an attractive route for the direct conversion of heat into electric power and is considered to be an important component of a sustainable future energy landscape. In fact, one need not invest too much into imagining the future of thermoelectric promise. As you read this, rovers on the planet Mars are creating kilometers worth of trails and roving the red planet using RTG technology as their primary power source. RTG can be unpacked as Radioisotope Thermoelectric Generator. RTG’s utilize the head from the natural decay of Plutonium.
The most common thermoelectric materials are alloys of chalcogenides such as Bi2Te3, PbTe, Bi2, and BixSb2−xTe3 are based on either bismuth telluride or lead telluride. These materials are relatively scarce and therefore expensive, toxic, and unstable at high temperatures. Transition-metal oxides were initially ignored in the search for potential thermoelectric materials until the discovery of high power factors in p-type NaxCoO2 about twenty years ago. Since then, many other metal oxides have been explored and reported as promising thermoelectric materials. Yet, the performance of most oxide materials is still lower than that of non-oxide traditional TE materials, and the effort is still ongoing in the search for efficient novel TE metal oxides.
The crystal structures of hexagonal perovskite oxides that were studied in Dr. Uzma’s research were studied using XRD, SXRD, and NPD at room temperature. Their crystal structure was heated without liquefaction (sintered) at a blistering temperature of 1150 °C. An increase in the crystalline size was noticed. This increase in the grain size, which was inferred from the diffraction data, and can be explained by the presence of Bismuth. The solid-state chemical reactions consist of four main steps of diffusion, reaction, nucleation, and crystal growth. When the diffusion rate is faster and the nucleus’s growth rate is greater than the nucleation rate at the given reaction conditions, larger crystals are formed. The low melting point of Bi2O3 (817 °C) compared to BaCO3 (1360 °C) suggests that the diffusion of Bi3+ cations will be faster than that of Ba2+ cations and, consequently, the crystallite/grain size will be larger in the Bismuth-doped samples for the given sintering time and temperature. Take a look at the electron micrographs obtained from this study.
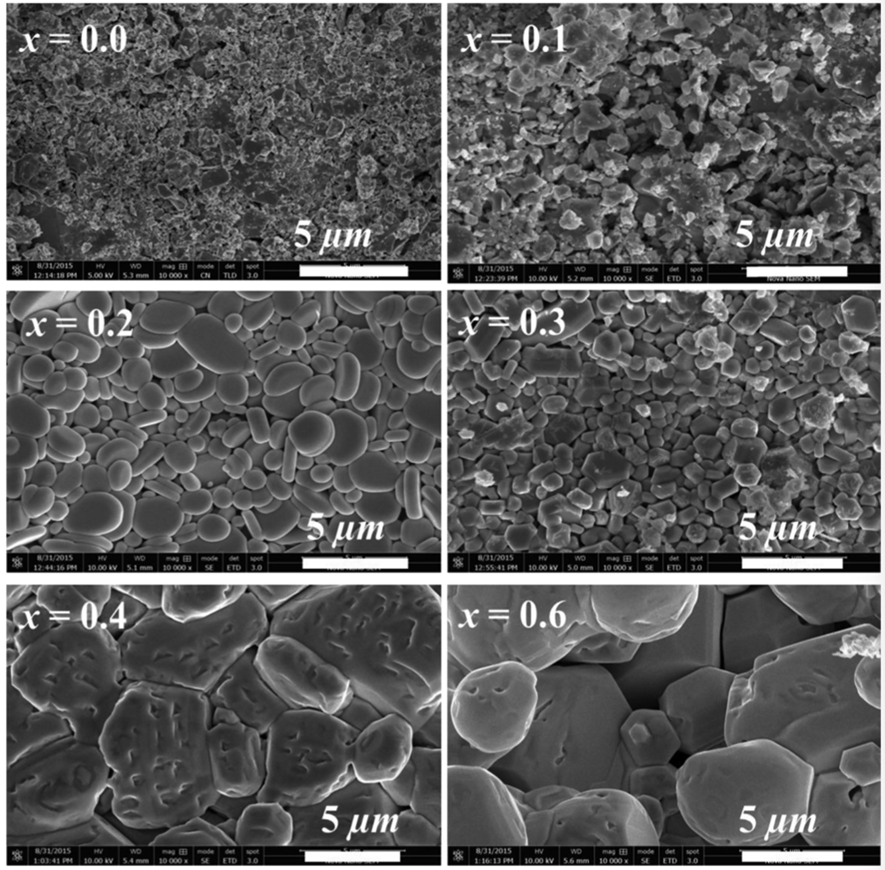
The researchers, including Dr. Falak Sher and Dr. Uzma Hira, have concluded that doping the perovskite crystal with Bismuth makes for a much better choice when it comes to selecting thermoelectric materials for harnessing energy from heat.
Read more about this work here:
Ba2–xBixCoRuO6 (0.0 ≤ x ≤ 0.6) Hexagonal Double-Perovskite-Type Oxides as Promising p-Type Thermoelectric Materials. Uzma Hira, Jan-Willem G. Bos, Alexander Missyul, François Fauth, Nini Pryds, and Falak Sher. Inorganic Chemistry 2021 60 (23), 17824-17836. DOI: 10.1021/acs.inorgchem.1c02442. Also see:
Recent Advances in Electrocatalysts toward Alcohol-Assisted, Energy-Saving Hydrogen Production

