Event date:
May
20
2021
4:00 pm
Catalytically active magnetic M@Fe3O4 core-shell nanoparticles via polymer-assisted surface confined metal nanoparticles growth approach
Supervisor
Dr. Basit Yameen
Student
Nazia Nawaz
Venue
Zoom Meetings (Online)
Event
MS Thesis defense
Abstract
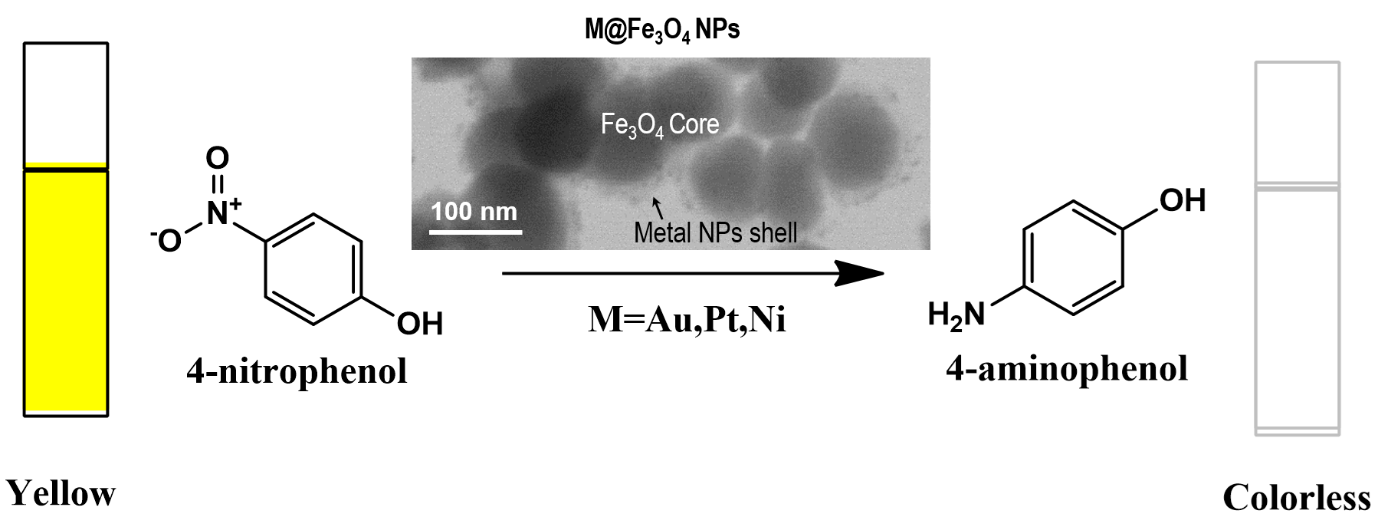
Magnetic nanoparticles (NPs) have attracted significant scientific interest because of their unique properties such as tunable magnetic behavior, high surface area and easy magnetic field assited separation. In the same vein, metal NPs are pushing the boundaries of science and engineering endeavors related to health, energy, environment, electronic and communication. In an attempt to combine the best of these two scientifically intriguing platforms, this project focused on the development of core-shell magnetically recoverable M@Fe3O4 NPs consisting of metal NPs shell (M= Au, Pt, Ni) and magnetic Fe3O4 NPs core via polymer assisted surface confined growth of metal NPs. In order to develop the targeted materials, polyethyleneimine (PEI) was functionalized on the surface of Fe3O4 NPs and the surface immobilized PEI was used to achieve the surface confined growth of metal NPs via two different approaches. In the first approach, metal ion chelating and reducing capacity and metal NPs stabilizing ability of the surface confined PEI was exploited to grow metal NPs on the surface of Fe3O4 NPs. In the second approach an external reducing agent was employed to assist in the surface confined growth of metal NPs. The M@Fe3O4 NPs obtained from both these approaches were fully characterized and their catalytic activities were evaluated. Scanning transmission electron microscopy (STEM) revealed that M@Fe3O4 NPs consisted of a core of Fe3O4 NPs (80-90 nm) with a shell of ultrasmall metal NPs. The catalytic activities of these magnetically separable M@Fe3O4 NPs were demonstrated using reduction of 4-nitrophenol to 4-aminophenol as a model reaction. The progress of the reduction reaction was monitored by measuring the change in UV-visible absorbance of a 20 mL (0.05 mM) aqueous solution of nitrophenol exposed to 2 mg of M@Fe3O4 NPs. All the M@Fe3O4 NP catalysts developed exhibited remarkable catalytic activities. Interestingly, the catalytic activities of all the M@Fe3O4 NPs remained unchanged over 10 recovery/reuse cycles. Our study suggests following order of catalytic activities of different M@Fe3O4 NP catalyst platforms developed in this study: Au@Fe3O4 NPs > Pt@ Fe3O4 NPs > Ni@ Fe3O4 NPs. In summary, we have developed a facile route to catalytically active and magnetically separable metal NPs functionalized magnetic NPs (M@Fe3O4 NPs) via PEI assisted surface confined growth of metal NPs. The materials platform developed in this work has the potential to contribute to the emerging concept of sustainability and circularity, particular in the domains of energy and environment.
Meeting Link: https://lums-edu-pk.zoom.us/j/94061435704?pwd=RFVDL3UvOUhHV0c0a2UxcFBHdDEyUT09
Meeting ID: 940 6143 5704
Password: 254387

