
N–Methylimidazole: A Versatile Reagent towards Organic Synthesis
Abstract:
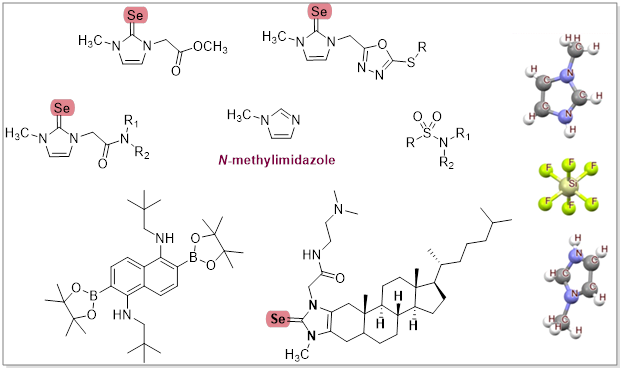
We chemists are dreamers. We think up new molecules and bring them to life" (Prof. Carolyn Bertozzi). In this spirit, this thesis investigates the synthesis of biologically relevant organoselenium and sulfur compounds using N–methylimidazole (NMI) as a versatile reagent. Chapter 1 details the synthesis of organoselenium compounds through the formation of an NHC–Se adduct and its transformation into a 1,3,4-oxadiazole-2-thiol scaffold, yielding 18 derivatives with diverse alkyl halides. Building on the versatile reactivity of the NHC–Se adduct explored in Chapter 1, Chapter 2 extends its value by synthesizing 15 amide derivatives and evaluating their potential anticancer properties, identifying 2.6A as a promising candidate. Its bioactivity, linked to a time-dependent proposed isomerization, was stronger in older batches, calling for further detailed investigation. Chapter 3 addresses the inefficiencies associated with directed electrophilic borylation. In this extensive project, a directed electrophilic approach was used to transform the directing group into a new functional group post-borylation, enabling the synthesis of functionalized organoboranes and ortho B-N-containing polyaromatic hydrocarbons. This transformation is facilitated by the directing group (amides), which stabilizes borenium cations. The significant Lewis acidity at the carbonyl center of these cations was effectively utilized to enable their in-situ reduction to amines using hydrosilanes. Chapter 4 proposes a selenium-modified cholesterol-based imidazolium salt (CHIM–L) to investigate cholesterol imbalance's role in Alzheimer’s disease. The synthesis involved modifying cholesterol with an imidazole moiety, followed by selenium incorporation, and finally introducing an amide moiety, yielding an artificial cholesterol mimic to study membrane processes associated with Alzheimer’s disease. Chapter 5 discloses the role of N–methylimidazole as a solvent, precatalyst, and as HF by-product scavenger in the green synthesis of aryl sulfonamides via Sulfur (VI) Fluoride Exchange (SuFEx) chemistry. The isolation and structural elucidation of the (often-overlooked) side product, 1-methyl-1H-imidazole-3-ium hexafluorosilicate (IV), underscore the significance of the glass vessel in the reaction mechanism, providing an alternative HF-free path for the silicon hexafluoride-based molten salts. NMI excels in its dual role of stabilizing silicon hexafluoride and providing insights into the reaction mechanism, further opening new avenues in organic synthesis.
Graphical Abstract:
Final Thesis Defense Committee:
Dr. Rahman Shah Zaib Saleem (FDC Convener, Thesis Committee Member, Associate Professor, LUMS)
Dr. Ghayoor Abbas Chotana (PhD Supervisor, Associate Professor, LUMS)
Dr. Muhammad Zaheer (Thesis Committee Member, Associate Professor, LUMS)
Dr. Amir Faisal (SBASSE Member, Associate Professor, LUMS)
Dr. Abdul Rauf Raza (External Examiner, Professor, Institute of Chemistry, University of Sargodha)
List of Publications:
Amides as modifiable directing groups in electrophilic borylation. Chem. Sci., 2023, 14, 3865. https://doi.org/10.1039/D2SC06483A
Imidazolium and benzimidazolium sulfonyl salts: Versatile functional group transfer reagents. Tetrahedron Lett., 2023, 128, 154696. https://doi.org/10.1016/j.tetlet.2023.154696
Sulfur (VI) Fluoride Exchange (SuFEx) via Glass–Assisted Organocatalysis. Accepted for publication in Adv. Synth. Catal. Published online on November 6, 2024. https://doi.org/10.1002/adsc.202401175
Synthesis and Characterization of NHC-Derived Amide-Functionalized Organoselenium Compounds (Under preparation)

